
Chemistry, 05.02.2023 09:40 geraldmorgan5580
How many kilojoules of heat are absorbed when 950 mL of water is heated from 27 degrees Celsius to 79 degrees Celsius? The specific heat of water is 4.18 J/g-C. Answer choices: 104 kJ 12 kJ 120 kJ 206 kJ
Answers

Answer from: jacquelineS7691
Answer
The kilojoules of heat absorbed = 206 kJ
Explanation
Given:
Volume of water = 950 mL
Initial temperature, T₁ = 27 ⁰C
Final temperature, T₂ = 79 ⁰C
Specific heat of water, c = 4.18 J/g ⁰C
What to find:
The kilojoules of heat absorbed.
Step-by-step solution:
Since density of water = 1 g/mL
Then the mass, m of water can be calculated first.
Density = Mass/Volume
⇒ Mass, m = Density x Volume = 1 g/mL x 950 mL = 950 g
Also, ΔT = T₂ - T₁ = 79 ⁰C - 27 ⁰C = 52 ⁰C
Therefore the kilojoules of heat absorbed, Q can be calculated using the formula given below
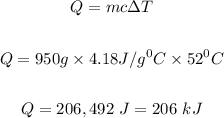
Therefore, the kilojoules of heat absorbed is 206 kJ

Answer from: Quest
Happy new years every one

Answer from: Quest
Change in speed or direction : )
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
How many kilojoules of heat are absorbed when 950 mL of water is heated from 27 degrees Celsius to 7...
Questions in other subjects:

Mathematics, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31


Medicine, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31






